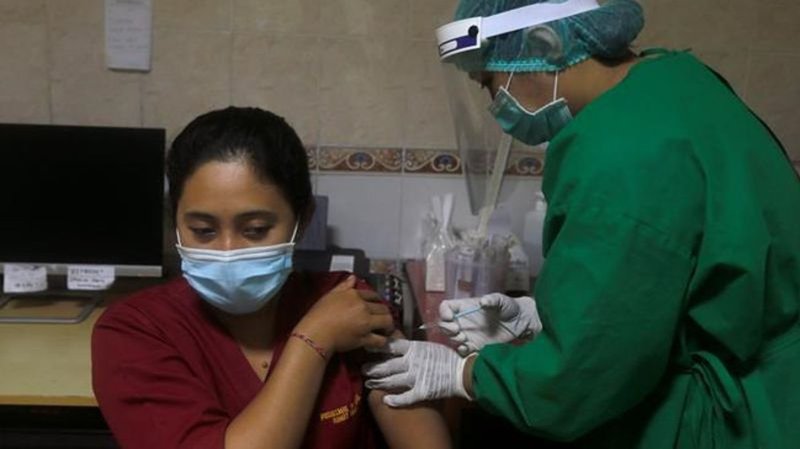
Indonesia green-lights emergency use of Chinese vaccine
JAKARTA, Indonesia — Indonesia’s Food and Drug Authority on Monday green-lighted emergency use of the COVID-19 vaccine produced by China-based Sinovac Biotech Ltd., with vaccinations of high-risk groups expected to start later this week.
Conditional vaccination of healthcare workers and other civil servants using the vaccine, called CoronaVac, is expected to begin this week.
“Based on the data and considering the guidance from (the World Health Organization), CoronaVac has met the requirements to get the permit to use the vaccine,” the chief of Indonesia Food and Drug Monitoring Agency, Penny Lukito, said at a news conference.
Indonesian President Joko Widodo said he would be the first person to receive the vaccine.


